|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lanthanides * |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Actinides ** |
|
|
|
|
electrons: |
|
|
|
|
|
|
||
|
Filled Levels |
|
|
|
|
|
|
||
|
Boron |
|
|
|
|||||
|
Carbon |
|
|
|
|||||
|
Nitrogen |
|
|
|
|||||
|
Oxygen |
|
|
|
|||||
|
Fluorine |
|
|
|
|||||
|
Aluminium |
|
|
|
|
|
|||
|
Silicon |
|
|
|
|
|
|||
|
Phosphorus |
|
|
|
|
|
|||
|
Sulphur |
|
|
|
|
|
|||
|
Chlorine |
|
|
|
|
|
Above is the Periodic Table of the Elements and the table shows the number of electrons in the outer most shells with yellow numbers (top). The elements in the center of the table (yellow backgrounds) are the transition metals and have additional outer layer electrons.
Another important type of bonding in molecules is "covalent" bonding. Covalent bonds are created when atoms share electrons between them. Remember atoms like to have filled electron energy levels and all electrons are more stable when paired.
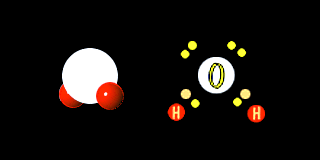
One of the most important compounds on earth is water. ( H2O or H-O-H ) Hydrogen shares its lone electron with oxygen. Oxygen needs two electrons to have a filled shell, it already has 6 electrons and needs two more.
Thus the water molecule has 8 total electrons in its out shell and both the oxygen and hydrogen are satisfied.
|
electrons: |
|
|
|
|
|
||
|
Oxygen |
|
|
|
||||
|
Hydrogen |
|
||||||
|
Hydrogen |
|
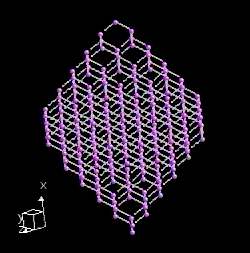
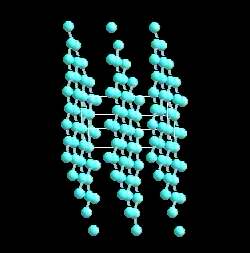
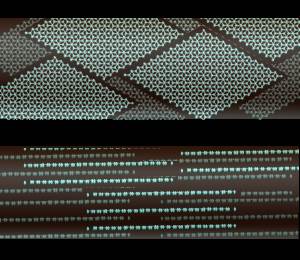
Graphite actually has two kinds of bonding present. It has shared electron bonds that are covalent. These are fundamentally the same as those in diamond and exist within the planes of hexagonal carbons. As you can see from the structure of graphite, it is made up of sheets of carbon with large spaces in-between.
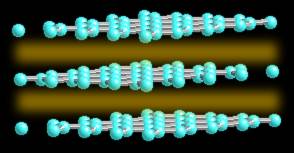
The spaces are held together with what is known as Van der Wahl bonding. It is a very weak bond. Because of is weakness carbon in the form of graphite is very strong in only one direction, and very weak in the second.
Have you ever head of "oriented graphite", it is the material used in space age plastics, in tennis rackets, golf clubs, and even in the space shuttle. By stretching graphite very thin, there are more of the strong planes across the narrow fibers than there are along the length and it grains strength. Although not really strong by itself, as a very thin fiber, it can be oriented in a polymer and held in place. Thus thousands of small strong overlapping strands are "oriented" in the fiber and they add strength to the polymer (called a composite).
| NEXT | TOC | PREV |