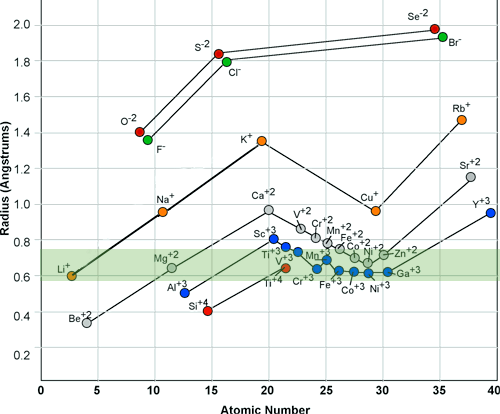
When an atom give up its outer electron or when it accepts electrons it becomes an ion. The ion is a charged atom that has an average radius in its charged state. Although each element in the periodic table has a different nucleus size, the majority of an atoms size is in the width of its outer most electronic shell.
The ionic radii is the effective size of the charged atom after it becomes a cation or anion. Different atoms may have similar ionic radii and when they do, they can easily substitute for each other in a crystal structure.
1.) It must be naturally formed. (No lab materials)
2.) It must be a solid. (No gas or liquid.)
3.) It must have a specific chemical composition.
(Elements or compounds are both covered by this definition. )
4.) It must have a characteristic crystal structure.
(Therefore no amorphous materials glass, amber, etc.)
Minerals have a crystal structure, so what is crystal structure? What does it mean? Originally crystal structure was defined by the faces on the crystals, their shape and form. The problem was that the same mineral often produced crystals with larger, smaller, longer, shorter, fatter, skinner, crystals. How could that be?
In 1669 the question was put to rest by Nicolaus Steno who uncovered the truth. It was not the faces that made a difference it was the ANGLES between the faces. Because the angles were always the same, he also reasoned that the internal structure must be consistent in each sample. The crystal habit, the characteristic form in which it most often grows can be used to aid in its identification.
Cleavage occurs when a mineral has a preferred direction of breakage. Cleavage like most other properties is a function of the crystal structure and the nature of the bonding. When a mineral is broken, it breaks into pieces that resemble one another, then it is said to have perfect cleavage.
Perfect cleavage is due to a higher order of symmetry and is more prevalent in minerals with strong ionic (therefore weak) bonding. Cleavage surfaces usually look almost polished and are very flat. The angles between cleavage surfaces is related to the crystal structure and hence diagnostic of the particular mineral.
Color is pretty simple, but unfortunately not a very good indicator. Many minerals have more than one common color, and this is due to low levels of impurities. Some minerals have a single mineral name and composition, but small variations are called by different names in some situations.
For example the mineral corundum (Al2O3) in a pure state is white or transparent, but if a small amount of the Al has been substituted by Cr then the color turns bright red and we call it ruby. If a little of the Al is replaced by Fe and Ti then it turns blue and we call it sapphire. Corundum may also be found in green, purple, pink, and yellow.
The mineral quartz can be found as a yellow crystal and is called "citrine", a purple crystal "amethyst", a pink crystal "rose quartz)", a brown-grey crystal "smoky, Cairngorm", or clear crystal and is called "rock crystal".
Some minerals after exposure to water or the atmosphere may under go surface color changes. So what you see may not be the actual color of the mineral. Sometimes a mineral can be stained by another. Quartz is often found with iron stains (red) on the surface.
 |
A streak is made by rubbing a mineral on a non-glaized porcelain plate. For instance hematite may look black, but it will always produce a RED/BROWN streak on a streak plate. Care must be taken if the mineral being tested is harder than the porcelain, the result will be a powder produced by the porcelain plate being scratched and will always be white. Pyrite looks yellow-gold in the crystal form, but gives a GRAY streak when the plate is used. Hematite on left |
| NEXT | TOC | PREV |