Cleavage
|
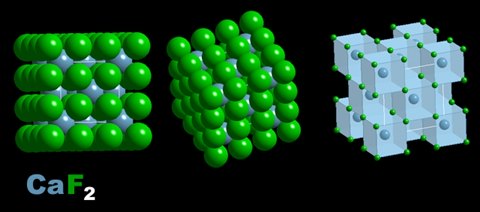
Cleavage and fracture are also related to the crystal structure of a mineral. Cleavage is the tendency of a mineral to break in straight line forming a clean, flat surface. Minerals with high levels of symmetry and weak elemental bonding tend to have excellent cleavage. Calcite (CaCO3), Fluorite (CaF2), Anhydrite (CaSO4) all have perfect cleavage. They have ionic (weak bonding) and belong to higher order (more symmetry) crystal groups. Diamond, although the hardest mineral with very good covalent bonding, also has perfect cleavage because it belongs to high symmetry group too. |
|
Notice the highly ordered and symmetrical lines of atoms in these structures. Combine that with ionic bonding and it is not surprising that these minerals are soft and have excellent cleavage. d covalent bonding, also has perfect cleavage because it belongs to high symmetry group too. |

|
|